The past several years have witnessed considerable changes in our overall healthcare system and research methodologies, presenting new opportunities and challenges. The complexity of evolving study designs and methods, privacy and security concerns, and regulatory aspects have grown substantially. Our ability to collate vast amounts of clinical and patient-reported outcomes has increased exponentially with electronic health records. Against this backdrop are increasing calls to prioritize research that generates results that are meaningful to patients and clinicians who care for them. The Research Management and Patient Integrated Data (RAPID) Core has been specifically created to facilitate clinical and translational research in this changing environment.
The overall goals of the RAPID Core are to streamline the process of human subjects research, integrate multiple sources of information, and foster patient-centered research.
The Specific Aims of the Core are to:
- Coordinate and oversee regulatory and operational aspects of human subjects research;
- Provide oversight and expertise to efficiently collect and export clinical and research data; and
- Facilitate patient-centered outcomes research (PCOR).
The RAPID Core consists of 3 Hubs (Research Management, Clinical Data Integration, and PCOR) to accomplish each of these aims. RAPID Core will create a robust enrichment program and provide guidance and resources to support state-of-the-science clinical and translation research to improve the lives of people living with rheumatic and autoimmune diseases.
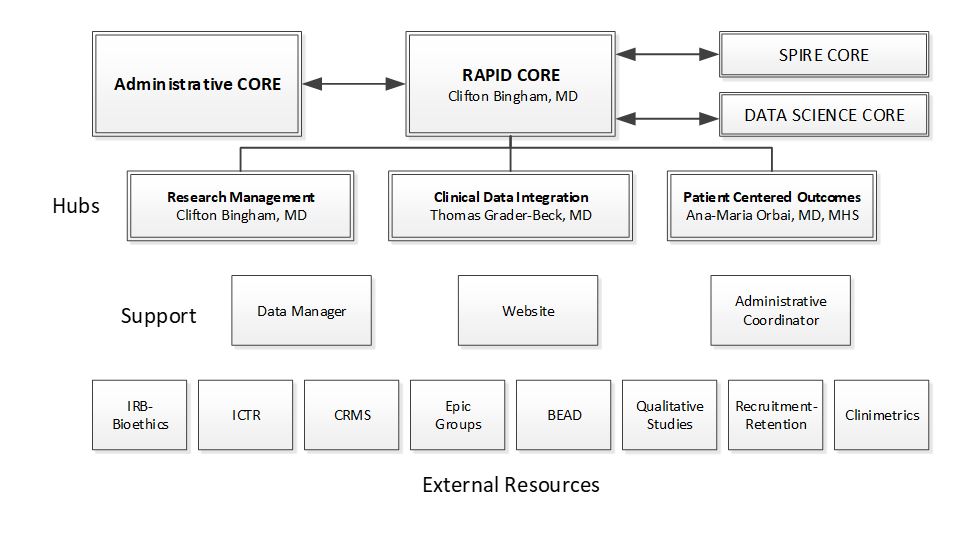
This RAPID Core has evolved from the Human Subjects and Data Analytical Core from the last RDRCC, which enabled harmonization of processes and oversight for research initiation, management, as well as helping to initiate and/or optimize study databases and cohort organization for efficiency and quality. This has become a critical support function across the entire Division, and has helped investigators new to rheumatology (e.g. new faculty from our own laboratories, Biomedical Engineering, Radiology, Pulmonary medicine) initiate projects related to rheumatic diseases within our Centers of Excellence and using other Core resources. A key feature of the RAPID Core has been its innovative approaches and attention to human subjects protections, oversight, and training for staff and faculty on changes in an increasingly complex regulatory framework; these efforts have been cited for their importance, and have been more broadly adopted at the institutional level. The RAPID Core includes a Clinical Data Management Integration Hub that is helping investigators integrate research within the EPIC electronic health record system and at the interface of care and research. These efforts are integral to the institutional inHealth initiative that aims to optimize the integration of data from multiple input sources to enable personalized care. To address the increasing recognition of incorporating the patient’s experience of disease and patient reported outcomes into comprehensive assessments, the RAPID Core offers a Human Subjects Research Hub providing a range of services and expertise for patient centered outcomes research to facilitate qualitative, participatory, and psychometric research
Research Management Hub (Clifton Bingham MD)
The complexity of human subjects research has increased substantially, with a greater appreciation of the need for privacy protection, ethical conduct of research, careful, systematic data acquisition, quality control, and enhanced security. Investigators face a number of additional requirements to initiate studies, including applications for review by institutional research boards, often leading to excessive delays that impair progress on projects. As datasets become more complex and are frequently linked with the acquisition of biological specimens from patients, there are increasing demands for improved data collection, biospecimen acquisition, and sample linkage to clinical data. The Research Management Hub led by Dr. Clifton Bingham and supported by two senior research staff provides expertise to investigators initiating and maintaining their projects. The Hub also maintains and regularly updates all research-related Standard Operating Procedures (SOPs). You may contact Michelle Jones, Data Manager (mrkjones@jhmi.edu, 410-550-9674) for more information about this Hub and its services
Data Management and Integration Hub (Thomas Grader-Beck MD)
Advances in health information technology have also provided new opportunities and challenges to investigators and clinicians. Traditionally much clinical research data was conducted on paper forms, but has quickly evolved to paperless systems along with the electronic health records for clinical care. Migration to these new systems requires specific expertise in data management, health information programming, and data security to enable effective transitions. Moreover, at an institutional level, Hopkins inHealth initiative has created a common clinical and research information management platform to facilitate personalized medicine approaches. In this quickly evolving arena, resources for the investigator to navigate the landscape and to migrate studies is increasingly necessary. The Core has developed a Data Management Hub to provide resources to investigators in this dynamic area.
Patient Centered Outcomes Research Hub (Ana Maria Orbai MHS MD)
Incorporating the patient’s voice into the research enterprise has been greatly emphasized to better understand the patient perspective and to improve patient health related quality of life. Qualitative methods are increasingly incorporated into research studies to obtain information from patients about their experience of disease through focus groups, interviews, and surveys. These methods help to understand the patient experience of their own illness and barriers and facilitators to care and better health outcomes. An evolution in psychometric methods has allowed the development of tools that enable the collection of highly validated and precise measures to quantify the patient experience of disease and that can greatly augment clinical data in defining precise disease subsets. The Patient Centered Research Hub provides access to expertise in qualitative and psychometric methods for investigators.


